PREPARATIVE CHROMATOGRAPHY
SYSTEMS
Ion Exchange System
Sanitech Engineers has supplied ion exchange columns and systems to addresses numerous separation and purification challenges within food, fermentation, biotechnology, and chemical industries. We are recognized experts in designing ion exchange and adsorption systems, developing processes, and performing resin screening to meet specific objectives. All our systems meet global regulatory compliance standards.
The ion chromatography separation process is based on ionic or electrostatic interactions between ionic and polar analytes, eluent ions, and ionic functional groups fixed to the chromatographic support. Ion chromatography separation involves two mechanisms: ion exchange through competitive ionic binding (attraction) and ion exclusion resulting from repulsion between similarly charged analyte ions and fixed support ions.
Ion exchange remains the most common ion chromatography type. This represents one of the most significant adsorption methods for separating charged molecules of varying molecular sizes and types, including peptides, proteins, nucleic acids, and related biopolymers. Separation occurs through ionic bonds between charged biomolecule groups and oppositely charged ion exchange gel or support. Since biomolecules have different charge characteristics, they interact with charged chromatographic media to varying degrees.
Separation is based on analyte binding to positively or negatively charged groups fixed on a stationary phase, which equilibrate with free counter ions in the mobile phase based on net surface charge variations.
Ion exchange chromatography, which is also known as adsorption chromatography, is a useful and popular method due to its:
- High capacity
- High resolving power
- Mild separation conditions
- Versatility and widespread applicability,
- Tendency to concentrate the sample
- Relatively low cost
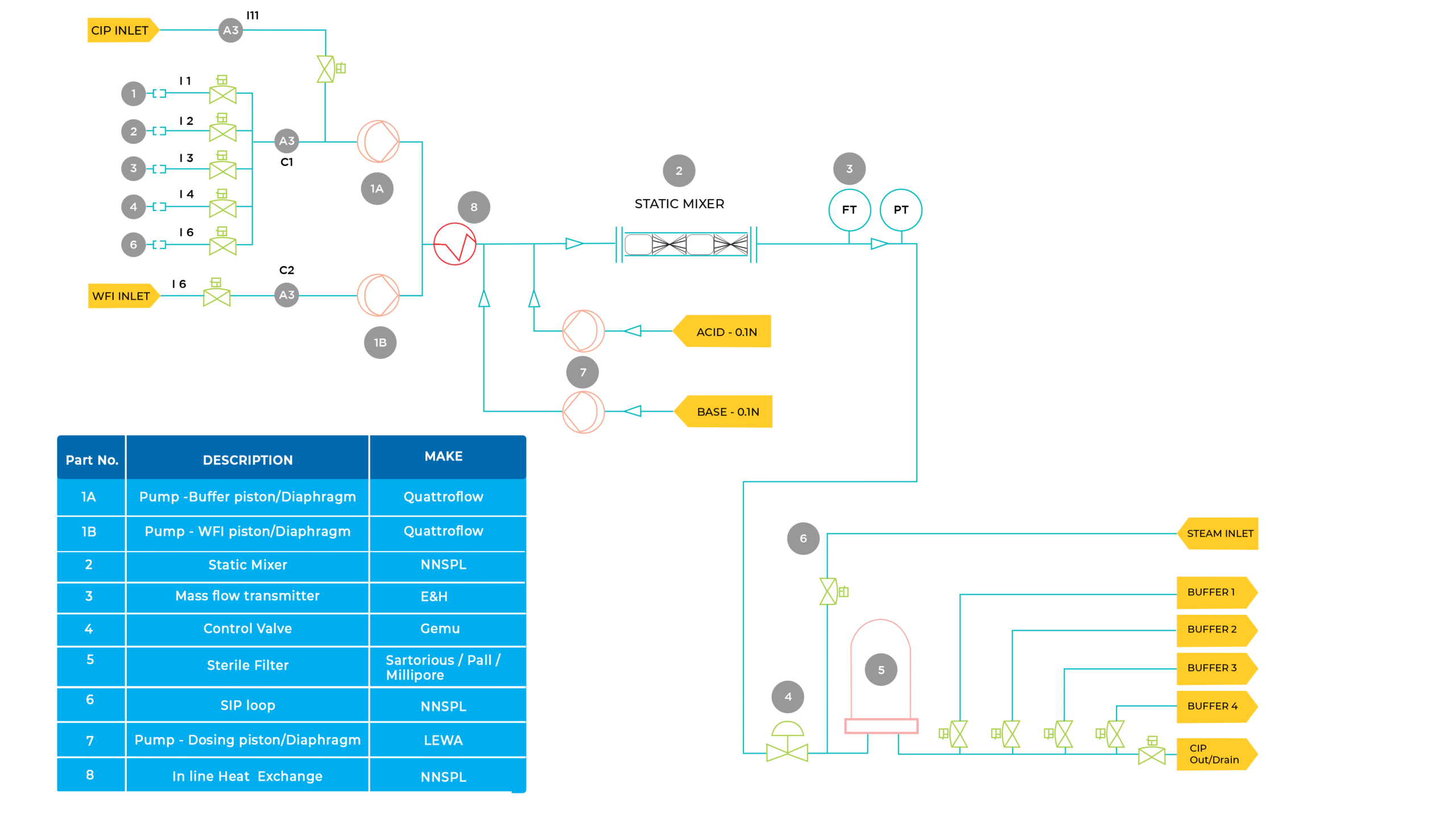
General components of an ion-exchange chromatography are:

High pressure pump
with pressure and flow indicator, to deliver the eluent
Injector
for introducing the sample into the eluent stream and into the column
Column
to separate the sample mixture into the individual components
Detector
to measure the analyte peaks as eluent from the column
Data system
for collecting and organizing the chromatograms and data
In ion-exchange chromatography, adsorption and desorption processes are determined by the properties of the three interacting entities:
- The stationary phase,
- The constituents of the mobile phase
- The solute
The expanding pharmaceutical sector and demand for highly specific, efficient separation techniques from specialty chemical and pharmaceutical industries have increased interest in liquid chromatographic technologies. Various liquid chromatography techniques are used to isolate bioactive compounds from diverse sources. Ion exchange chromatography is probably the most powerful and traditional liquid chromatography type, still widely used alone or combined with other chromatographic techniques for analyzing and separating ionizable molecules or those with different charges, including proteins, enzymes, peptides, amino acids, nucleic acids, carbohydrates, polysaccharides, and lectins.
Ion exchange chromatography also separates and purifies organic compounds from natural sources, such as deprotonated acids like fatty acids or amino acid derivatives, or protonated bases like alkaloids. Ion exchange chromatography offers numerous advantages: extensive application to large numbers of molecules with high capacity, cost-effectiveness, easy scalability to factory scales, high target molecule purification degrees, and solvent-free natural product extraction capability.
Consequently, ion exchange chromatography, employed for over 50 years to separate ionic molecules, remains a practical and popular technique for natural product isolation in modern drug discovery, continuing to grow as new technologies develop.